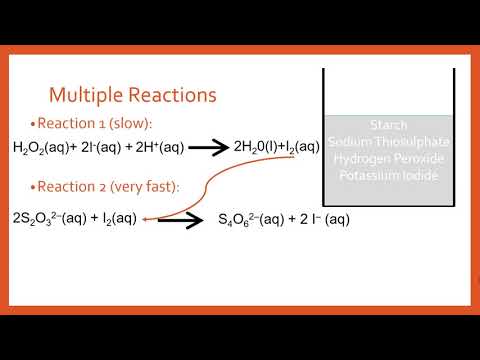
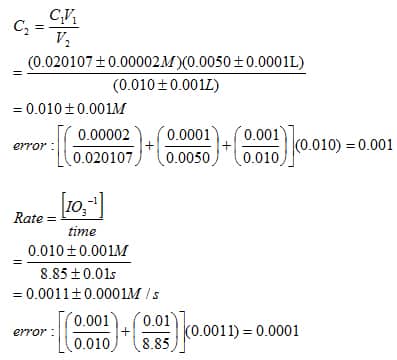
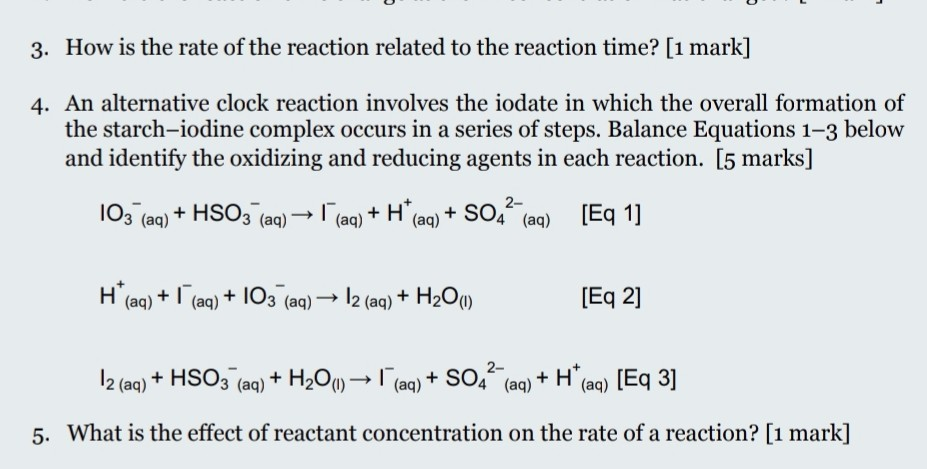
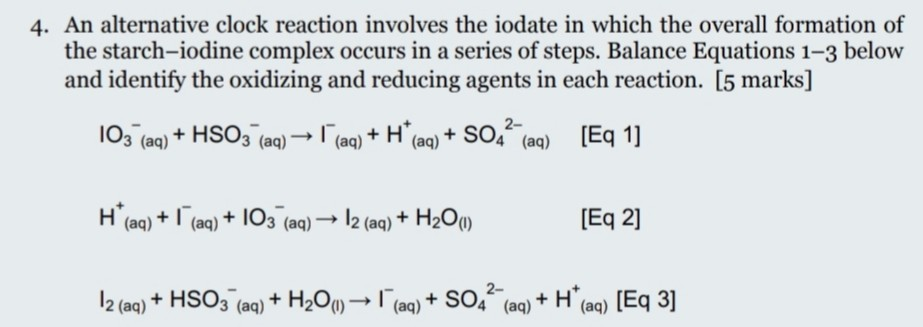
SOLVED: Instructions: Begin by watching this video: Iodine Clock Reaction You will see two different reactions slow reaction in which Iodide ions are produced and fast reaction in which in which the
What is the reaction order with respect to [S2O8 2-] and [I-] in a persulfate-iodide reaction? - Quora
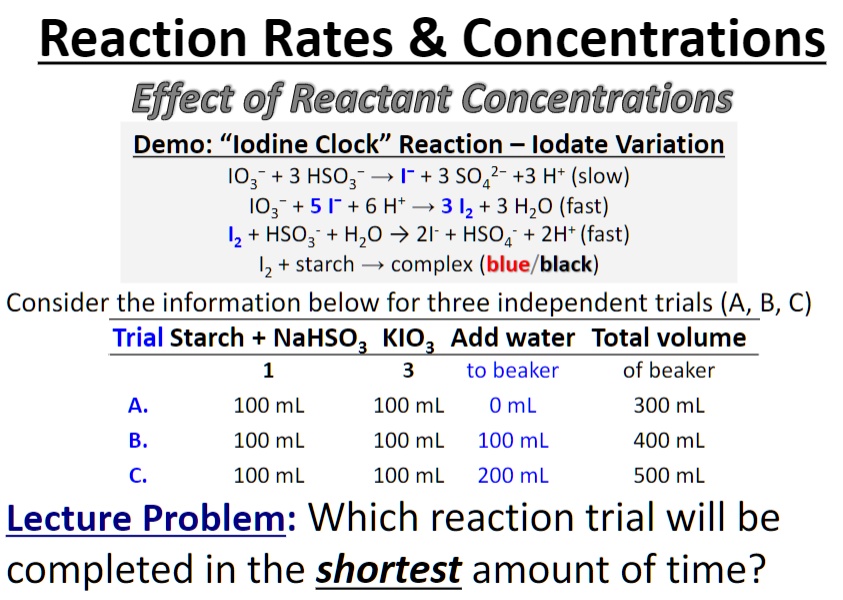
SOLVED: Reaction Rates Concentrations Effect of Reactant Concentrations Demo: "lodine Clock" Reaction lodateVariation I03 + 3 HSO3 > 1-+ 3 S042-+3 Ht (slow) I03 +51 + 6 Ht> 3 12 + 3
![Rate Law Equation For the reaction: aX + bY products r = k [X] m [Y] n Where r = reaction rate k = rate constant [X] & [Y] = concentration Rate Law Equation For the reaction: aX + bY products r = k [X] m [Y] n Where r = reaction rate k = rate constant [X] & [Y] = concentration](https://images.slideplayer.com/25/8124398/slides/slide_5.jpg)
Rate Law Equation For the reaction: aX + bY products r = k [X] m [Y] n Where r = reaction rate k = rate constant [X] & [Y] = concentration
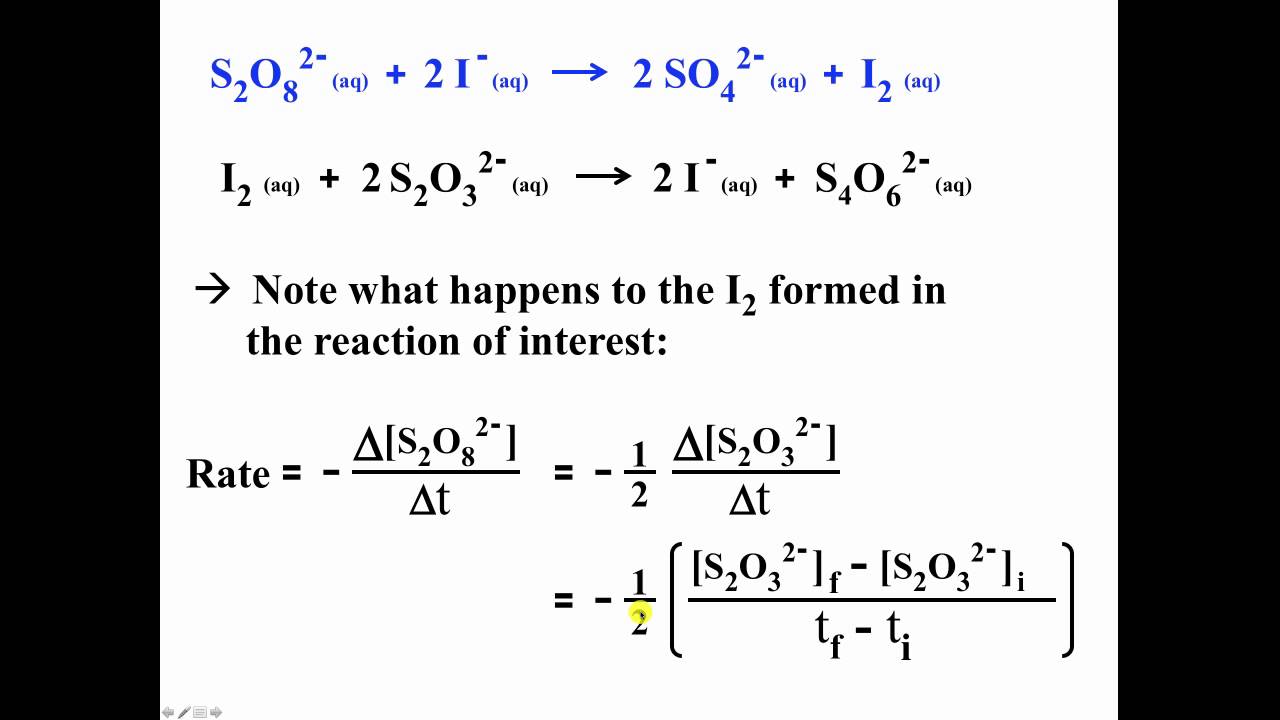
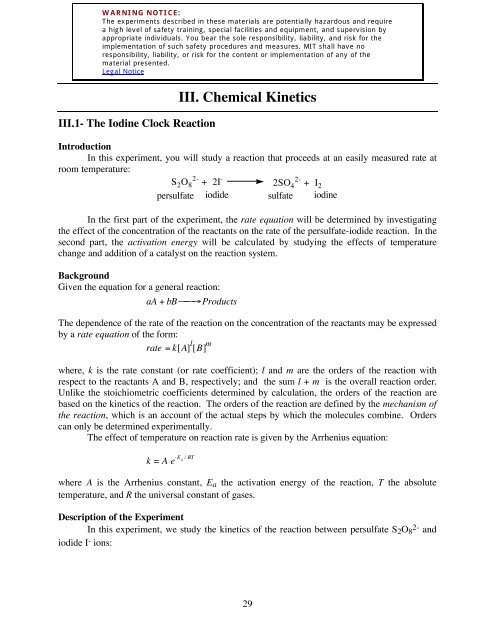
Chemical Kinetics: The Iodine-Clock Reaction: S2O8 (aq) → I2(aq) + 2 SO4 2 S2O3 This reaction is much faster than the previ

SOLVED: proposed mechanism for the iodine clock reaction is shown below: Iodine Clock Reaction Mechanism IO3 + HSO3 S0 + HIO2 k =2.95 x 10-'M-Is-1 HIO2 + I- + H+ + 2HOI

Iodine Clock Reaction We will begin by describing a proposed reaction mechanism for the iodine clock reaction. There are several variations to this reaction, - ppt download
![SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3- ] [I- ] 2[H+]^2a)This reaction is third order with respect to H+.b)This reaction is first order with SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3- ] [I- ] 2[H+]^2a)This reaction is third order with respect to H+.b)This reaction is first order with](https://cdn.numerade.com/ask_previews/494c958b-2794-4a7f-861f-022f5f445939_large.jpg)
SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3- ] [I- ] 2[H+]^2a)This reaction is third order with respect to H+.b)This reaction is first order with

162 Lab Iodine Clock Reaction 2014 0227 GF1 - Lab Manual | Clock Reaction Reaction Kinetics: The - Studocu

A Closer Examination of the Mechanism of the Hydrogen Peroxide Iodine-Clock Reaction with Respect to the Role of Hypoiodite Species | Journal of Chemical Education

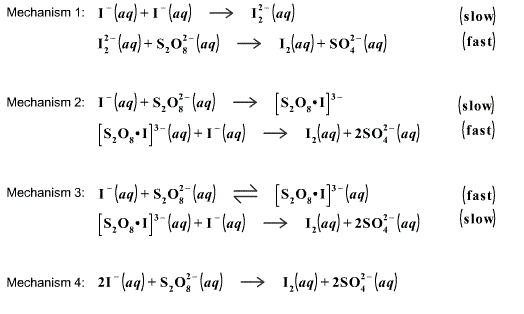
![How to do lab report [Exp 004] Rates of Reaction for Iodine Clock Reaction - YouTube How to do lab report [Exp 004] Rates of Reaction for Iodine Clock Reaction - YouTube](https://i.ytimg.com/vi/L1CtBY_xmZs/maxresdefault.jpg)